News
Many questions raised by medical experts on Sinopharm unanswered by its manufacturer

by Suresh Perera
The report of the advisory panel of medical experts, seen by The Sunday Island, raised some critical questions on the “safety, efficacy and immunogenicity” of the Sinopharm vaccine.
The report says there was no response from the Chinese manufacturer on how the vaccine induces antibodies (neutralizing antibodies and IgG antibodies to the SARS-CoV2) compared to the responses following natural infection. i.e. antibody responses induced by the vaccine in comparison to antibodies convalescent serum following natural infection. The manufacturer only provided seroconversion rates of the two vaccine arm.
The panel was of the view that it was important to find out if the immune responses elicited by the vaccine are adequate. All other vaccines showed a higher or an equal antibody response compared to natural infection.
There was also no answer provided to neutralizing antibody levels in those over 60 years of age in comparison to younger individuals. The manufacturer only provided Seroconversion rates of 18-59 and 60 but not the neutralizing antibody levels. It was vital to elicit a response to this to determine the immunogenicity of this vaccine in older individuals, the report said.
The following were the questions raised by the experts and the response given (or not given, as in some cases) by the manufacturer:
Q:
The lack of detectable SARS-CoV2 IgG antibodies 14 days after the first dose and also very minimal at 28 days (when they received the 2nd dose).
Answer
by the manufacturer: Higher titres of antibodies were induced following the second dose than the first.
(The panel observed that SARS-CoV2 inactivated vaccine produced by a different manufacturer, high levels of antibodies were seen at 28 days following a single dose. A good antibody response has been observed with the inactivated vaccines. inactivated polio vaccine after one dose, which is boosted by the second and third doses. The levels of antibodies following inactivated vaccines is lower but the levels are still detectable after a single dose).
Q:
Interim analysis of phase 3 data presented until October 31. There was no data after that. Can the follow up data be provided?
(Not answered)
(The panel noted that as the participants would have been followed after October 31, 2020, it would be important to have more safety and efficacy data. Such follow up data related to other vaccines have been made available through phase III clinical trials reports published in peer-reviewed journals).
Q:
Vaccine efficacy is claimed to be 76.06% and 78.01%. Were participants only followed up for an average period of 22 days after the second dose?
(Not answered)
Observation by Panel: Period of follow up is insufficient.
Q:
Efficacy data in 60 year old age group. The sample size inadequate to draw conclusions.
(Not answered)
Observation by Panel: Since this is the most vulnerable group for COVID-19 infection as well as severe disease, this data is required.
Q:
Was anyone with comorbidities included in the trial?
(Not answered)
Observation by Panel: Data required to determine efficacy and safety in those with comorbidities. In the exclusion criteria of the trial, it appears that all those with comorbidities have been excluded from study.
Q:
Phase 3 safety data. What were the side effects observed? Only the percentage of AE given and no breakdown of the type of side effects seen with the two vaccines. What are the type of grade 3 and 4 side effects observed and the proportion who experienced each side effect?
(Not answered)
Observation by Panel: Detailed information on types AEs is important to make an assessment on safety of the vaccine. The safety data should be available in an age-specific manner.
Q:
Some people had itching. Did anyone develop allergies or anaphylaxis? Rashes with itching? What are the ingredients of the vaccine? Does it have BSA or FBS? Since vero cells are known to be grown in FBS if there is contamination that might cause issues in those with beef allergy.
(Not answered).
Observation by Panel: As above.
Q:
How many were included in the analysis of immunogenicity? How many from all age groups? When reporting the GMTs of neutralizing Abs and binding antibodies, only median/mean have been reported. No idea about the range, IQR or SD.
(Not answered).
Observation by Panel: This information is critical to make an assessment of immunogenicity of the vaccine. The number of individuals in whom immunogenicity was evaluated is also important.
Q:
T cell studies. To show whether vaccine activates a TH1 response, rather than a TH2.
Manufacturer has noted that relevant studies have not been conducted.
Observation by Panel: In previous clinical trials on inactivated vaccines for measles and RSV, and also animal studies on SARS, a TH2 response caused organ pathology, including deaths, after infection with wild-type virus. This was attributed to a TH2 response, rather than the ideal TH1.
News
Cabinet approval to prepare new Act for securing the rights of plant species

Although there are legal provisions for the right to publish, technical planning, right of patent, trademarks and enterprises etc, in the Intellectual Property Act No. 36 of 2003, there are no provisions for securing the rights of plant species (relevant to breeders, researchers and farmers).
In addition, no patents can be issued for flora and fauna according to the provisions of the intellectual rights act. Therefore, approval of the Cabinet of Ministers was granted at their meeting held on 22.05.2024 to introduce a new act for securing the rights of plant species.
Wherefore, the Legal Draftsman has pointed out that policy approval of the new Cabinet of Ministers should be taken to complete the drafting of the new flora species rights securing act.
Accordingly, the Cabinet of Ministers granted approval to the proposals submitted by the Minister of Agriculture, Livestock, Lands and Irrigation for the preparation of the said draft bill.
Latest News
National minimum monthly salary for private sector employees raised to Rs. 27,000/-, minimum daily wage to Rs. 1080/-

Parallel to salary hike of public sector officers by the budget proposals 2025, it has been proposed to increase the salaries of private sector employees as well.
Accordingly, the Cabinet of Ministers granted approval to the proposal submitted by the Minister of Labour to revise the national minimum monthly salary and national minimum daily wage and follow other legal actions as follows:
• To raise the minimum national monthly salary by rupees 9,500/- from rupees 17,500/- to rupees 27,000/- with effect from 01.04.2025
• To raise the minimum national daily salary by rupees 380/- from rupees 700/- to rupees 1,080/- with effect from 01.04.2025
• To raise the minimum national monthly salary by rupees 3,000/- from rupees 27,000/- to rupees 30,000/- with effect from 01.01.2026
• To raise the minimum national daily salary by rupees 120/- from rupees 1,080 /- to rupees 1,200/- with effect from 01.01.2026
News
Draft bill to establish the Chartered Institute of Media Professionals – Sri Lanka to be completed

The Cabinet of Ministers approved the proposal furnished by the Minister of Health and Mass Media to grant policy approval of the current Cabinet of Ministers for completion of formulating the draft bill to establish the Chartered Institute of Media Professionals – Sri Lanka
Establishment of the Chartered Institute of Media Professionals of Sri Lanka has been recognized in order to accomplish the requirement of a training institute for carrying out relevant studies with the objective of presenting media intellects fortified with skills for the uplifting quality and standards of media society of this country while creating chartered professional journalists.
-

 Business2 days ago
Business2 days agoColombo Coffee wins coveted management awards
-

 Features2 days ago
Features2 days agoStarlink in the Global South
-

 Business4 days ago
Business4 days agoDaraz Sri Lanka ushers in the New Year with 4.4 Avurudu Wasi Pro Max – Sri Lanka’s biggest online Avurudu sale
-

 Business5 days ago
Business5 days agoStrengthening SDG integration into provincial planning and development process
-
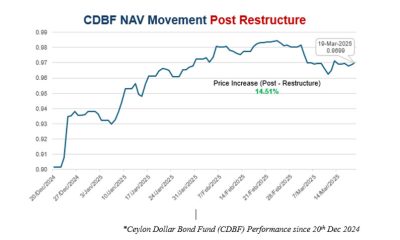
 Business4 days ago
Business4 days agoNew SL Sovereign Bonds win foreign investor confidence
-

 Sports5 days ago
Sports5 days agoTo play or not to play is Richmond’s decision
-

 Features2 days ago
Features2 days agoModi’s Sri Lanka Sojourn
-

 Sports5 days ago
Sports5 days agoNew Zealand under 85kg rugby team set for historic tour of Sri Lanka











