Features
Ratmalana airport’s operations and unplanned development

Wellington Airport, New Zealand, Control Tower, in a shopping Mall
By Capt G A Fernando MBA
gafplane@sltnet.lk
President, Aircraft Owners and Operators Association
RCyAF, Air Ceylon, Air Lanka, SIA, SriLankan Airlines
In 1934 the State Council of Ceylon decided that an airport with easy access to Colombo was a necessity and declared that Ratmalana was the best site available. Accordingly an airfield was built and the first landing took place on 27 November 1935. This was Ceylon’s first Airfield. With the advent of WW II, the airport expanded and a runway was also built. The Royal Air Force (RAF) and the Fleet Air Arm used it.
To assist aircraft landing in bad weather and resulting bad visibility, a transmitter was built at Thalangama to generate a Radio Signal Beam directed along the extended centre line of the runway at Ratmalana. If the aircraft was tracking the correct path, the pilots would hear a continuous tone on their head sets. However if they were left of the desired track, the pilots would hear a letter ‘A’ in Morse code (dit dah) or if right, a letter ‘N’ which was a (dah dit) in Morse. The objective was to hear a continuous signal in the ears, which guided them towards Ratmalana. The decent to lower altitude was at the pilot’s discretion.
 As time went by, there was a low frequency, Non Directional Radio Beacon (NDB) placed in the vicinity of the airport (Attidiya) and used with an Automatic Direction Finder (ADF) on board the aircraft. A needle over a compass dial pointed to Attidiya and gave directional guidance to international air traffic. Although it could accommodate an unlimited number of aircraft, when they needed it most like during thunderstorms activity, there was static interference and the needles pointed towards the thunderstorms instead of Attidiya. So a Very High Frequency Omni Directional Radio Range known as a ‘VOR’ for short was installed for more accuracy.
As time went by, there was a low frequency, Non Directional Radio Beacon (NDB) placed in the vicinity of the airport (Attidiya) and used with an Automatic Direction Finder (ADF) on board the aircraft. A needle over a compass dial pointed to Attidiya and gave directional guidance to international air traffic. Although it could accommodate an unlimited number of aircraft, when they needed it most like during thunderstorms activity, there was static interference and the needles pointed towards the thunderstorms instead of Attidiya. So a Very High Frequency Omni Directional Radio Range known as a ‘VOR’ for short was installed for more accuracy.
Then in 1968, Ratmalana International Airport lost its ‘International’ status when the Bandaranaike International Airport opened and all international operations moved to Katunayake. The equipment at the airport was allowed to deteriorate. The Radio Navigational let down aids ran down. There was no proper Control Tower. Even the runway lights were not working and night flights had to depend on Kerosene Lamps. One redeeming grace, in the night, in those days, was that the Sapugaskanda Oil Refinery was in full production and the giant flare of the burning gasses was the guiding light to the Ratmalana Airport. The Pilots spotted the flare from far away and flew over the Refinery and then turned on the runway heading and could see the runway edge kerosene flares, flickering dimly in a dark patch that was the Ratmalana airport! The civil training aircraft of the Government Flying School of those days had neither radios nor any radio aids to navigation.
Post 1977, after the ‘Dharmista’ Government created another problem for Ratmalana operations. The authorities decided to build a new Capital in Sri Jayewardenepura, Kotte, and also move the New Parliament to that neck of the woods. Unfortunately, the Parliament was just 3.6 Nautical Miles from the end of the Ratmalana runway ‘as the crow flies’ and less than 1NM from the Thalangama Transmitters. In most countries overflying the Parliament is prohibited. Therefore the Authorities blindly decreed the same in Sri Lanka. Thus restricting the freedom of aircraft movements to the Ratmalana Runway and preventing safer, conventional landing approaches. It must be noted that Air Ceylon and other domestic flights were still using Ratmalana. Many professionals were quick to observe that it was akin to someone building a house near a railway line and then complaining that it was too noisy and requiring the railway to divert!
Everyone had learnt to live with the non-availability of precision Navigational aids at Ratmalana. Thalangama Transmitters lost its significance. The Urban Development Authority (UDA) eventually, took vacant procession and the SL Army (Gemunu watch) established a camp there. During December, with clear nights and cooler mornings, (Temperature inversions) combined with the North Easterly winds blowing smoke from the Sapugaskanda Refinery, the visibility on the final approach tends to get very bad at the Ratmalana airport.
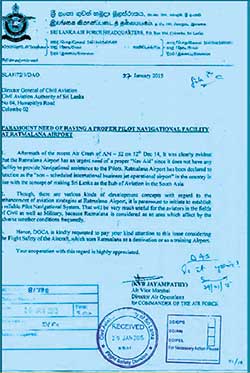
In fact, on the morning of 14 December 2014, a SLAF Antonov AN 32, ferry flight from Katunayake, attempted to approach for landing at Ratmalana and crashed killing the crew. This prompted the then Air Force Commander to write to the then Director General of Civil Aviation (DGCA) to reinstall Navigational Facilities (See Letter below). Now almost five years have gone by and at last the Airport and Aviation Sri Lanka (AASL) is slowly waking up to the fact that not only the seen but also the unseen facilities at Ratmalana should be brought up to standard in the name of air safety. Wide publicity is given to the fact that the Government’s intention is to make Ratmalana an International Business aircraft hub and regain its lost glory.
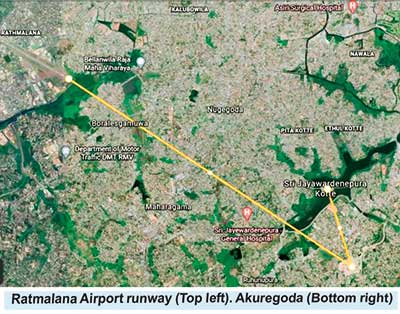 While this concept is being actively pursued by the Airport and Aviation Sri Lanka Ltd (AASL) and the Civil Aviation Authority Sri Lanka (CAASL), another security sensitive building has been erected in the vacant land at Thalangama, barely 1 NM from the Parliament and 4.4 NM from the runway end. That being the Akuregoda Military Headquarters. This has created an effective barrier for operation of legitimate air traffic. As a direct result more restrictions are bound to be imposed on inbound air traffic. Furthermore, it does seem like that there was no ‘master plan’ and the left hand didn’t know what the right hand was doing.
While this concept is being actively pursued by the Airport and Aviation Sri Lanka Ltd (AASL) and the Civil Aviation Authority Sri Lanka (CAASL), another security sensitive building has been erected in the vacant land at Thalangama, barely 1 NM from the Parliament and 4.4 NM from the runway end. That being the Akuregoda Military Headquarters. This has created an effective barrier for operation of legitimate air traffic. As a direct result more restrictions are bound to be imposed on inbound air traffic. Furthermore, it does seem like that there was no ‘master plan’ and the left hand didn’t know what the right hand was doing.
Air safety dictates that jet planes should have at least an eight mile straight in (no turns) final approach. Now it is not possible to do that with the unplanned sensitive buildings on the final approach to Ratmalana. Ideally, like in other countries, all three parties, the Local Municipality Town Planer, the Civil Aviation Authority/ Airport and Aviation Ltd (CAASL/ AASL), and the Building developer, must make these long term decisions. In Australia for instance the CAA’ Airports Authority has control of manmade obstacles for a radius of 25 miles. Unplanned buildings, called ‘man made relief’ as against ‘Geographic Relief’ (terrain) has spoilt the feasibility of the intended City Airport. Another case in point is the Kotalawala Defence University (K DU). Which is the tallest building in the vicinity of the airport that should never have been allowed to be built that high. This seems to be the malady this whole country is suffering from. The people in the know are afraid to speak. Subsequently, no one is held accountable for these poor, uncoordinated decisions. The true professionals are not consulted. As a result vision is ‘tunnelled’. The Sinhala saying “Leda malath, bada suddai” seems to be very appropriate. (Although the patient died, the bowels were clean!)
It is believed that accommodating Business Jets inbound to Ratmalana airport will be a money spinner and a step in the correct direction. There is no point in crying over spilt milk one would say. What is done is done. I write to offer a practical solution.to mitigate the adverse effects of unplanned buildings. While the Military Base is working 24/7 (around the clock) and overflying prohibition may be justified, the Parliament ‘works’ only on certain days and for limited hours. The Authorities should provisionally allow air traffic, inbound to Ratmalana to overfly the Parliament on days and times that there are no sittings. A Notice to Airmen (NOTAM) could be issued as and when necessary. In other words let’s share the airspace for the benefit of all, without attempting to kill the goose that may lay the golden egg. Then longer and therefore safer approaches could be designed to facilitate these small but fast Business Jets which will be operating in and out of the Ratmalana Airport.
The Ratmalana Airport lacks a proper Control Tower with a 360 degree visibility of the area. A new Control Tower could be sited in the highest point in the vicinity. Perhaps at KDU to mitigate the hopeless situation. The Wellington, New Zealand Control Tower is on top of a Shopping Mall!
To put this in the correct perspective, according to the FAA (Federal Aviation Administration) USA, research shows that light training aircraft and other small aircraft of the size and mass of business jets cannot create catastrophic destruction to strong buildings like our Parliament or the Military Base, like what happened on 9/11 with large passenger jets. Some even say that the Pentagon was damaged by a missile and not a passenger aircraft. But that’s another story. 
Features
Lasting solutions require consensus

Problems and solutions in plural societies like Sri Lanka’s which have deep rooted ethnic, religious and linguistic cleavages require a consciously inclusive approach. A major challenge for any government in Sri Lanka is to correctly identify the problems faced by different groups with strong identities and find solutions to them. The durability of democratic systems in divided societies depends less on electoral victories than on institutionalised inclusion, consultation, and negotiated compromise. When problems are defined only through the lens of a single political formation, even one that enjoys a large electoral mandate, such as obtained by the NPP government, the policy prescriptions derived from that diagnosis will likely overlook the experiences of communities that may remain outside the ruling party. The result could end up being resistance to those policies, uneven implementation and eventual political backlash.
A recent survey done by the National Peace Council (NPC), in Jaffna, in the North, at a focus group discussion for young people on citizen perception in the electoral process, revealed interesting developments. The results of the NPC micro survey support the findings of the national survey by Verite Research that found that government approval rating stood at 65 percent in early February 2026. A majority of the respondents in Jaffna affirm that they feel safer and more fairly treated than in the past. There is a clear improving trend to be seen in some areas, but not in all. This survey of predominantly young and educated respondents shows 78 percent saying livelihood has improved and an equal percentage feeling safe in daily life. 75 percent express satisfaction with the new government and 64 percent believe the state treats their language and culture fairly. These are not insignificant gains in a region that bore the brunt of three decades of war.
Yet the same survey reveals deep reservations that temper this optimism. Only 25 percent are satisfied with the handling of past issues. An equal percentage see no change in land and military related concerns. Most strikingly, almost 90 percent are worried about land being taken without consent for religious purposes. A significant number are uncertain whether the future will be better. These negative sentiments cannot be brushed aside as marginal. They point to unresolved structural questions relating to land rights, demilitarisation, accountability and the locus of political power. If these issues are not addressed sooner rather than later, the current stability may prove fragile. This suggests the need to build consensus with other parties to ensure long-term stability and legitimacy, and the need for partnership to address national issues.
NPP Absence
National or local level problems solving is unlikely to be successful in the longer term if it only proceeds from the thinking of one group of people even if they are the most enlightened. Problem solving requires the engagement of those from different ethno-religious, caste and political backgrounds to get a diversity of ideas and possible solutions. It does not mean getting corrupted or having to give up the good for the worse. It means testing ideas in the public sphere. Legitimacy flows not merely from winning elections but from the quality of public reasoning that precedes decision-making. The experience of successful post-conflict societies shows that long term peace and development are built through dialogue platforms where civil society organisations, political actors, business communities, and local representatives jointly define problems before negotiating policy responses.
As a civil society organisation, the National Peace Council engages in a variety of public activities that focus on awareness and relationship building across communities. Participants in those activities include community leaders, religious clergy, local level government officials and grassroots political party representatives. However, along with other civil society organisations, NPC has been finding it difficult to get the participation of members of the NPP at those events. The excuse given for the absence of ruling party members is that they are too busy as they are involved in a plenitude of activities. The question is whether the ruling party members have too much on their plate or whether it is due to a reluctance to work with others.
The general belief is that those from the ruling party need to get special permission from the party hierarchy for activities organised by groups not under their control. The reluctance of the ruling party to permit its members to join the activities of other organisations may be the concern that they will get ideas that are different from those held by the party leadership. The concern may be that these different ideas will either corrupt the ruling party members or cause dissent within the ranks of the ruling party. But lasting reform in a plural society requires precisely this exposure. If 90 percent of surveyed youth in Jaffna are worried about land issues, then engaging them, rather than shielding party representatives from uncomfortable conversations, is essential for accurate problem identification.
North Star
The Leader of the Lanka Sama Samaja Party (LSSP), Prof Tissa Vitarana, who passed away last week, gave the example for national level problem solving. As a government minister he took on the challenge the protracted ethnic conflict that led to three decades of war. He set his mind on the solution and engaged with all but never veered from his conviction about what the solution would be. This was the North Star to him, said his son to me at his funeral, the direction to which the Compass (Malimawa) pointed at all times. Prof Vitarana held the view that in a diverse and plural society there was a need to devolve power and share power in a structured way between the majority community and minority communities. His example illustrates that engagement does not require ideological capitulation. It requires clarity of purpose combined with openness to dialogue.
The ethnic and religious peace that prevails today owes much to the efforts of people like Prof Vitarana and other like-minded persons and groups which, for many years, engaged as underdogs with those who were more powerful. The commitment to equality of citizenship, non-racism, non-extremism and non-discrimination, upheld by the present government, comes from this foundation. But the NPC survey suggests that symbolic recognition and improved daily safety are not enough. Respondents prioritise personal safety, truth regarding missing persons, return of land, language use and reduction of military involvement. They are also asking for jobs after graduation, local economic opportunity, protection of property rights, and tangible improvements that allow them to remain in Jaffna rather than migrate.
If solutions are to be lasting they cannot be unilaterally imposed by one party on the others. Lasting solutions cannot be unilateral solutions. They must emerge from a shared diagnosis of the country’s deepest problems and from a willingness to address the negative sentiments that persist beneath the surface of cautious optimism. Only then can progress be secured against reversal and anchored in the consent of the wider polity. Engaging with the opposition can help mitigate the hyper-confrontational and divisive political culture of the past. This means that the ruling party needs to consider not only how to protect its existing members by cloistering them from those who think differently but also expand its vision and membership by convincing others to join them in problem solving at multiple levels. This requires engagement and not avoidance or withdrawal.
by Jehan Perera
Features
Unpacking public responses to educational reforms

 As the debate on educational reforms rages, I find it useful to pay as much attention to the reactions they have excited as we do to the content of the reforms. Such reactions are a reflection of how education is understood in our society, and this understanding – along with the priorities it gives rise to – must necessarily be taken into account in education policy, including and especially reform. My aim in this piece, however, is to couple this public engagement with critical reflection on the historical-structural realities that structure our possibilities in the global market, and briefly discuss the role of academics in this endeavour.
As the debate on educational reforms rages, I find it useful to pay as much attention to the reactions they have excited as we do to the content of the reforms. Such reactions are a reflection of how education is understood in our society, and this understanding – along with the priorities it gives rise to – must necessarily be taken into account in education policy, including and especially reform. My aim in this piece, however, is to couple this public engagement with critical reflection on the historical-structural realities that structure our possibilities in the global market, and briefly discuss the role of academics in this endeavour.
Two broad reactions
The reactions to the proposed reforms can be broadly categorised into ‘pro’ and ‘anti’. I will discuss the latter first. Most of the backlash against the reforms seems to be directed at the issue of a gay dating site, accidentally being linked to the Grade 6 English module. While the importance of rigour cannot be overstated in such a process, the sheer volume of the energies concentrated on this is also indicative of how hopelessly homophobic our society is, especially its educators, including those in trade unions. These dispositions are a crucial part of the reason why educational reforms are needed in the first place. If only there was a fraction of the interest in ‘keeping up with the rest of the world’ in terms of IT, skills, and so on, in this area as well!
Then there is the opposition mounted by teachers’ trade unions and others about the process of the reforms not being very democratic, which I (and many others in higher education, as evidenced by a recent statement, available at https://island.lk/general-educational-reforms-to-what-purpose-a-statement-by-state-university-teachers/ ) fully agree with. But I earnestly hope the conversation is not usurped by those wanting to promote heteronormativity, further entrenching bigotry only education itself can save us from. With this important qualification, I, too, believe the government should open up the reform process to the public, rather than just ‘informing’ them of it.
It is unclear both as to why the process had to be behind closed doors, as well as why the government seems to be in a hurry to push the reforms through. Considering other recent developments, like the continued extension of emergency rule, tabling of the Protection of the State from Terrorism Act (PSTA), and proposing a new Authority for the protection of the Central Highlands (as is famously known, Authorities directly come under the Executive, and, therefore, further strengthen the Presidency; a reasonable question would be as to why the existing apparatus cannot be strengthened for this purpose), this appears especially suspect.
Further, according to the Secretary to the MOE Nalaka Kaluwewa: “The full framework for the [education] reforms was already in place [when the Dissanayake government took office]” (https://www.wsws.org/en/articles/2025/08/12/wxua-a12.html, citing The Morning, July 29). Given the ideological inclinations of the former Wickremesinghe government and the IMF negotiations taking place at the time, the continuation of education reforms, initiated in such a context with very little modification, leaves little doubt as to their intent: to facilitate the churning out of cheap labour for the global market (with very little cushioning from external shocks and reproducing global inequalities), while raising enough revenue in the process to service debt.
This process privileges STEM subjects, which are “considered to contribute to higher levels of ‘employability’ among their graduates … With their emphasis on transferable skills and demonstrable competency levels, STEM subjects provide tools that are well suited for the abstraction of labour required by capitalism, particularly at the global level where comparability across a wide array of labour markets matters more than ever before” (my own previous piece in this column on 29 October 2024). Humanities and Social Sciences (HSS) subjects are deprioritised as a result. However, the wisdom of an education policy that is solely focused on responding to the global market has been questioned in this column and elsewhere, both because the global market has no reason to prioritise our needs as well as because such an orientation comes at the cost of a strategy for improving the conditions within Sri Lanka, in all sectors. This is why we need a more emancipatory vision for education geared towards building a fairer society domestically where the fruits of prosperity are enjoyed by all.
The second broad reaction to the reforms is to earnestly embrace them. The reasons behind this need to be taken seriously, although it echoes the mantra of the global market. According to one parent participating in a protest against the halting of the reform process: “The world is moving forward with new inventions and technology, but here in Sri Lanka, our children are still burdened with outdated methods. Opposition politicians send their children to international schools or abroad, while ours depend on free education. Stopping these reforms is the lowest act I’ve seen as a mother” (https://www.newsfirst.lk/2026/01/17/pro-educational-reforms-protests-spread-across-sri-lanka). While it is worth mentioning that it is not only the opposition, nor in fact only politicians, who send their children to international schools and abroad, the point holds. Updating the curriculum to reflect the changing needs of a society will invariably strengthen the case for free education. However, as mentioned before, if not combined with a vision for harnessing education’s emancipatory potential for the country, such a move would simply translate into one of integrating Sri Lanka to the world market to produce cheap labour for the colonial and neocolonial masters.
According to another parent in a similar protest: “Our children were excited about lighter schoolbags and a better future. Now they are left in despair” (https://www.newsfirst.lk/2026/01/17/pro-educational-reforms-protests-spread-across-sri-lanka). Again, a valid concern, but one that seems to be completely buying into the rhetoric of the government. As many pieces in this column have already shown, even though the structure of assessments will shift from exam-heavy to more interim forms of assessment (which is very welcome), the number of modules/subjects will actually increase, pushing a greater, not lesser, workload on students.

A file photo of a satyagraha against education reforms
What kind of education?
The ‘pro’ reactions outlined above stem from valid concerns, and, therefore, need to be taken seriously. Relatedly, we have to keep in mind that opening the process up to public engagement will not necessarily result in some of the outcomes, those particularly in the HSS academic community, would like to see, such as increasing the HSS component in the syllabus, changing weightages assigned to such subjects, reintroducing them to the basket of mandatory subjects, etc., because of the increasing traction of STEM subjects as a surer way to lock in a good future income.
Academics do have a role to play here, though: 1) actively engage with various groups of people to understand their rationales behind supporting or opposing the reforms; 2) reflect on how such preferences are constituted, and what they in turn contribute towards constituting (including the global and local patterns of accumulation and structures of oppression they perpetuate); 3) bring these reflections back into further conversations, enabling a mutually conditioning exchange; 4) collectively work out a plan for reforming education based on the above, preferably in an arrangement that directly informs policy. A reform process informed by such a dialectical exchange, and a system of education based on the results of these reflections, will have greater substantive value while also responding to the changing times.
Two important prerequisites for this kind of endeavour to succeed are that first, academics participate, irrespective of whether they publicly endorsed this government or not, and second, that the government responds with humility and accountability, without denial and shifting the blame on to individuals. While we cannot help the second, we can start with the first.
Conclusion
For a government that came into power riding the wave of ‘system change’, it is perhaps more important than for any other government that these reforms are done for the right reasons, not to mention following the right methods (of consultation and deliberation). For instance, developing soft skills or incorporating vocational education to the curriculum could be done either in a way that reproduces Sri Lanka’s marginality in the global economic order (which is ‘system preservation’), or lays the groundwork to develop a workforce first and foremost for the country, limited as this approach may be. An inextricable concern is what is denoted by ‘the country’ here: a few affluent groups, a majority ethno-religious category, or everyone living here? How we define ‘the country’ will centrally influence how education policy (among others) will be formulated, just as much as the quality of education influences how we – students, teachers, parents, policymakers, bureaucrats, ‘experts’ – think about such categories. That is precisely why more thought should go to education policymaking than perhaps any other sector.
(Hasini Lecamwasam is attached to the Department of Political Science, University of Peradeniya).
Kuppi is a politics and pedagogy happening on the margins of the lecture hall that parodies, subverts, and simultaneously reaffirms social hierarchies.
Features
Chef’s daughter cooking up a storm…
 Don Sherman was quite a popular figure in the entertainment scene but now he is better known as the Singing Chef and that’s because he turns out some yummy dishes at his restaurant, in Rajagiriya.
Don Sherman was quite a popular figure in the entertainment scene but now he is better known as the Singing Chef and that’s because he turns out some yummy dishes at his restaurant, in Rajagiriya.
However, now the spotlight is gradually focusing on his daughter Emma Shanaya who has turned out to be a very talented singer.
In fact, we have spotlighted her in The Island a couple of times and she is in the limelight, once gain.
When Emma released her debut music video, titled ‘You Made Me Feel,’ the feedback was very encouraging and at that point in time she said “I only want to keep doing bigger and greater things and ‘You Made Me Feel’ is the very first step to a long journey.”
Emma, who resides in Melbourne, Australia, is in Sri Lanka, at the moment, and has released her very first Sinhala single.
“I’m back in Sri Lanka with a brand new single and this time it’s a Sinhalese song … yes, my debut Sinhala song ‘Sanasum Mawana’ (Bloom like a Flower).
“This song is very special to me as I wrote the lyrics in English and then got it translated and re-written by my mother, and my amazing and very talented producer Thilina Boralessa. Thilina also composed the music, and mix and master of the track.”
Emma went on to say that instead of a love song, or a young romance, she wanted to give the Sri Lankan audience a debut song with some meaning and substance that will portray her, not only as an artiste, but as the person she is.
Says Emma: “‘Sanasum Mawana’ is about life, love and the essence of a woman. This song is for the special woman in your life, whether it be your mother, sister, friend, daughter or partner. I personally dedicate this song to my mother. I wouldn’t be where I am right now if it weren’t for her.”
On Friday, 30th January, ‘Sanasum Mawana’ went live on YouTube and all streaming platforms, and just before it went live, she went on to say, they had a wonderful and intimate launch event at her father’s institute/ restaurant, the ‘Don Sherman Institute’ in Rajagiriya.
It was an evening of celebration, good food and great vibes and the event was also an introduction to Emma Shanaya the person and artiste.
Emma also mentioned that she is Sri Lanka for an extended period – a “work holiday”.
“I would like to expand my creativity in Sri Lanka and see the opportunities the island has in store for me. I look forward to singing, modelling, and acting opportunities, and to work with some wonderful people.
“Thank you to everyone that is by my side, supporting me on this new and exciting journey. I can’t wait to bring you more and continue to bloom like a flower.”
-

 Life style3 days ago
Life style3 days agoMarriot new GM Suranga
-

 Business2 days ago
Business2 days agoMinistry of Brands to launch Sri Lanka’s first off-price retail destination
-

 Features3 days ago
Features3 days agoMonks’ march, in America and Sri Lanka
-

 Midweek Review7 days ago
Midweek Review7 days agoA question of national pride
-

 Business7 days ago
Business7 days agoAutodoc 360 relocates to reinforce commitment to premium auto care
-

 Opinion6 days ago
Opinion6 days agoWill computers ever be intelligent?
-

 Features3 days ago
Features3 days agoThe Rise of Takaichi
-

 Features3 days ago
Features3 days agoWetlands of Sri Lanka:













