News
Presidential poll cannot be put off through referendum – Prof. Peiris

Dissident SLPP MP Prof. G. L. Peiris yesterday (19) said it was not possible to postpone a presidential election with the help of a referendum.
The former External Affairs Minister explained how the law, pertaining to the referendum, couldn’t be exploited by the government to achieve its political objectives.
Addressing the media at his Kirula Road residence, Prof. Peiris said that presidential election would have to be conducted between Sept 17 and Oct 17 this year and the new President sworn in before Nov 17.
“We are confident the election will be held as scheduled,” the ex-Minister said, reminding the Election Commission that it would be its responsibility to issue an official statement as regards the scheduled poll in late July or early August this year.
Prof. Peiris said that the presidential poll couldn’t be put off through a 2/3 majority in Parliament. But that, too, wouldn’t be possible, the MP said, adding that abolition of executive presidency couldn’t be used to postpone national elections.
The former top law academic said that such vital constitutional reforms should be undertaken by an executive and Parliament properly elected by the electorate.
Prof. Peiris said that Ranil Wickremesinghe, who couldn’t retain his Colombo seat, was not acceptable as the person to lead constitutional reforms.
Responding to media queries, Prof. Peiris said that his group would back SJB leader Sajith Premadasa who could win the next election.
Prof. Peiris said that the government was in the process of strengthening anti-riot police in a bid to intimidate the Opposition. The ex-Minister revealed new equipment ordered by police headquarters to meet public protests while warning the Opposition couldn’t be silenced by what he called offensive laws such as the recently enacted Online Safety Bill and the proposed Anti-Terrorism Law (SF)
Latest News
Advisory for Heavy Rain issued for the Central, Uva, Sabaragamuwa, Eastern and North-central provinces and in Galle and Matara districts
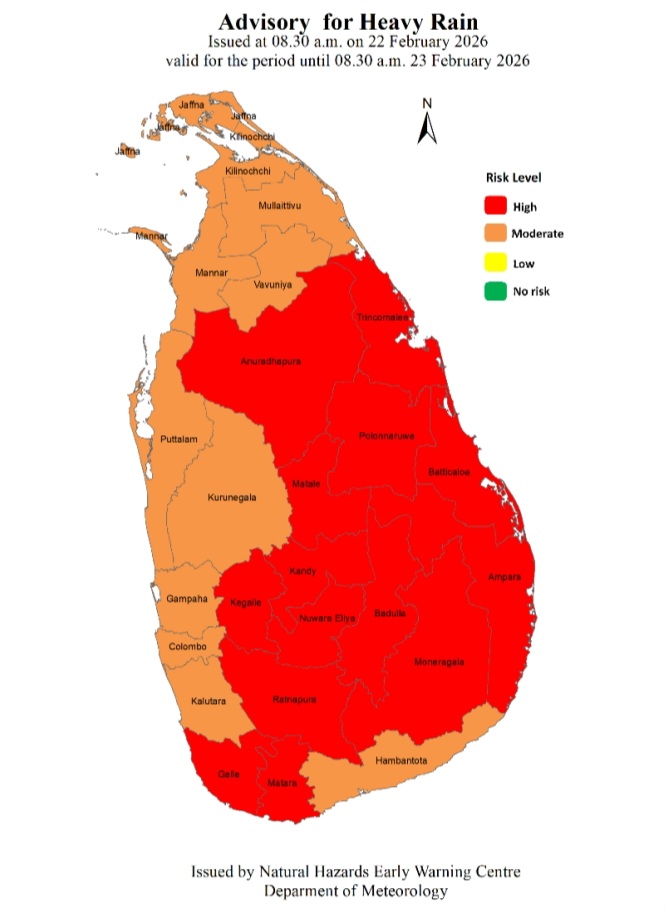
Advisory for Heavy Rain Issued by the Natural Hazards Early Warning Centre at 08.30 a.m. on 22 February 2026 valid for the period until 08.30 a.m. 23 February 2026
Due to the influence of the low level atmospheric disturbance in the vicinity of Sri Lanka, Heavy showers above 100 mm are likely at some places in Central, Uva, Sabaragamuwa, Eastern and North-central provinces and in Galle and Matara districts.
Therefore, general public is advised to take adequate precautions to minimize damages caused by heavy rain, strong winds and lightning during thundershowers
News
Matara Festival for the Arts’ inaugurated by the Prime Minister

The inaugural ceremony of the Matara Festival for the Arts, featuring a wide range of creations by local and international artists, was held on February 19 at the Old High Court premises of the Matara Fort, under the patronage of Prime Minister Dr. Harini Amarasuriya.
The festival, centred around the Old High Court premises in Matara and the auditorium of the Matara District Secretariat, will be open to the public from 20 to 23 of February. The festival will be featured by visual art exhibitions, short film screenings, Kala Pola, and a series of workshops conducted by experts.
The inaugural event was attended by the Minister of Women and Child Affairs, Ms. Saroja Paulraj, along with artists, guests, and a large number of schoolchildren.
(Prime Minister’s Media Division)
News
Only single MP refuses salary as Parliament details pays and allowances

Only one Member of Parliament has chosen not to receive the salaries and allowances entitled to MPs, Prime Minister Dr. Harini Amarasuriya revealed in Parliament last Thursday, shedding light on the financial perks enjoyed by members of the Tenth Parliament.
Speaking on Thursday (Feb. 19) in response to a question from SJB Badulla District MP Chaminda Wijesiri, the Prime Minister outlined the full range of pay and allowances provided to parliamentarians.
According to Dr. Amarasuriya, MPs receive a monthly allowance of Rs. 54,285, an entertainment allowance of Rs. 1,000, and a driver’s allowance of Rs. 3,500—though MPs provided with a driver through the Ministry of Public Security and Parliamentary Affairs are not eligible for the driver’s allowance.
Additional benefits include a telephone allowance of Rs. 50,000, a transport allowance of Rs. 15,000, and an office allowance of Rs. 100,000. MPs are also paid a daily sitting allowance of Rs. 2,500 for attending parliamentary sessions, with an additional Rs. 2,500 per day for participation in parliamentary sittings and Rs. 2,500 per day as a committee allowance.
Committee meetings held on non-parliament sitting days also attract Rs. 2,500 per day.
Fuel allowances are provided based on the distance between an MP’s electoral district and Parliament. National List MPs are entitled to a monthly allocation equivalent to 419.76 litres of diesel at the market price on the first day of each month.
Despite the comprehensive benefits, only SJB Badulla District MP Nayana Wasalathilaka has opted not to draw a salary or allowances. Dr. Amarasuriya said that in accordance with a written notification submitted by MP Wasalathilaka on August 20, 2025, payments have been suspended since that date.
The Prime Minister also confirmed that she, along with the Speaker, Deputy Speaker, committee chairs, ministers, deputy ministers, the Opposition Leader, and senior opposition whips, have all informed the Secretary-General of Parliament in writing that they will not claim the fuel allowance.
Challenging the ruling party’s voluntary pledge to forgo salaries, MP Wijesiri pointed out that all MPs except Wasalathilaka continue to receive their salaries and allowances. “On one hand you speak about the people’s mandate, which is good. But the mandate also included people who said they would voluntarily serve in this Parliament without salaries. Today we have been able to prove, Hon. Speaker, that except for one SJB MP, the other 224 Members are drawing parliamentary salaries,” he said.
The Prime Minister responded by defending the political culture and practice of allocating portions of MPs’ salaries to party funds. Referring to previous practices by the JVP and NPP, she said: “It is no secret to the country that the JVP has for a long time not personally taken MPs’ salaries or any allowances. I think the entire country knows that these go to a party fund. That is not new, nor is it something special to mention. The NPP operates in the same way. That too is not new; it is the culture of our political movement.”
When MP Wijesiri posed a supplementary question asking whether diverting salaries to party funds was an indirect method of taking care of MPs, Dr. Amarasuriya said: “There is no issue there. No question was raised; the Member made a statement. What we have seen throughout this week is an inability to understand our political culture and practice, and a clash with decisions taken by political movements that misused public funds. What is coming out is a certain mindset. That is why there is such an effort to find fault with the 159. None of these facts are new to people. He did not ask a question, so I have nothing to answer.”
The disclosures come days after the Government moved to abolish the parliamentary pension, a measure that has sparked renewed debate over MP compensation and the transparency of funds allocation.
-

 Business6 days ago
Business6 days agoMinistry of Brands to launch Sri Lanka’s first off-price retail destination
-

 Features10 hours ago
Features10 hours agoWhy does the state threaten Its people with yet another anti-terror law?
-

 Latest News2 days ago
Latest News2 days agoNew Zealand meet familiar opponents Pakistan at spin-friendly Premadasa
-

 Latest News2 days ago
Latest News2 days agoTariffs ruling is major blow to Trump’s second-term agenda
-

 Latest News2 days ago
Latest News2 days agoECB push back at Pakistan ‘shadow-ban’ reports ahead of Hundred auction
-

 Features6 days ago
Features6 days agoGiants in our backyard: Why Sri Lanka’s Blue Whales matter to the world
-

 Sports3 days ago
Sports3 days agoOld and new at the SSC, just like Pakistan
-

 News2 days ago
News2 days agoConstruction begins on country’s largest solar power project in Hambantota